Revenue Cycle Management
Reimbursement should be a forethought, planned
during assay validation.
Does your institution need help navigating the reimbursement landscape of your NGS project?
Guarantee Financial & Clinical Success
Reimbursement strategy based on region & assay
Planning for Medicare coverage by region
MolDx dossiers & tech assessment support
Assay launch preparation support
Expert consultation every step of the way
Plan Reimbursement Before Launching Your Program

Expert Consultation
- Medicare MAC/Payer Mix Analysis
- Review & Optimize Prior Authorization Workflow
- Payor Templates: Pre-authorizations, appeals by level, letter of medical necessity
- Expert Consultation: ICD-10 coding options & CLFS Coding Options by Assay
- Up to 150 hours of total project support

Collaboration on MolDx Packages & Market Access Dossiers
- Analytical Validity, Clinical Validation Summary for NGS Solid Tumors or Myeloid Malignancies or Hereditary Germline testing cancer panels
- Technical Assessment Submission Checklist and Questionnaire
- Validation Sample Level Data Spreadsheet Master List

Assay Launch Preparation Support
- Assay Overview, Description & Summary Creation by Assay
- Patient Financial Assistance Program Review
- Sub Panels/Disease Specific Tests Review
- IT/RCM Integration Overview
- Payor Contracting Review
- Pricing Review

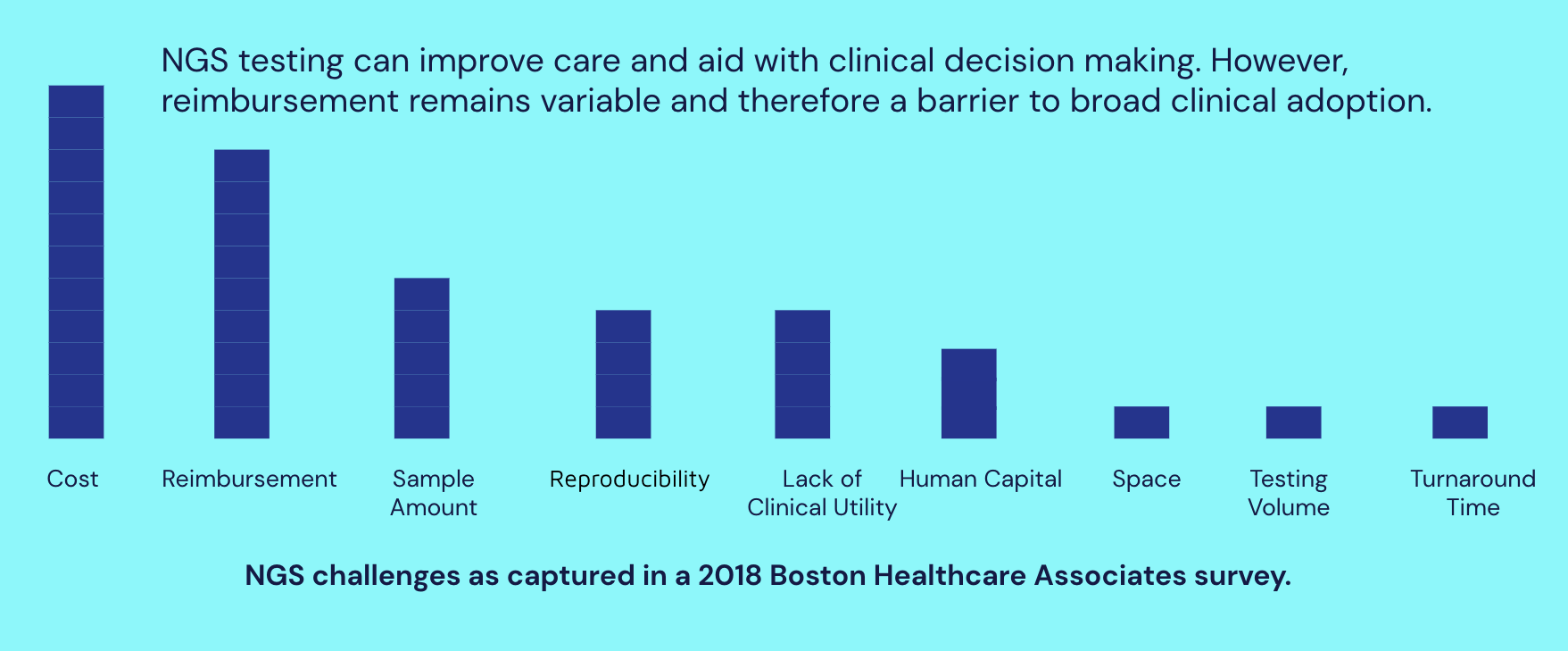
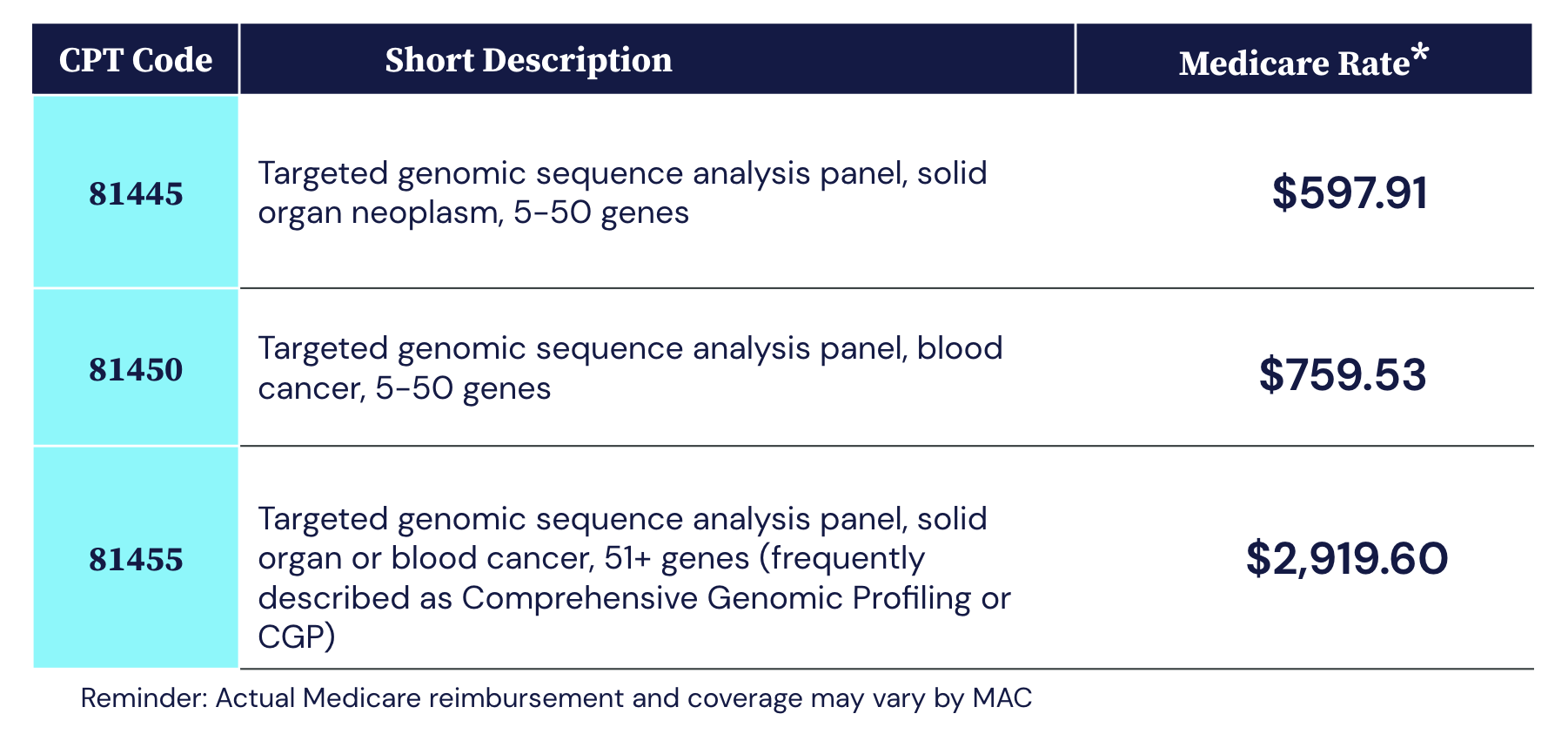
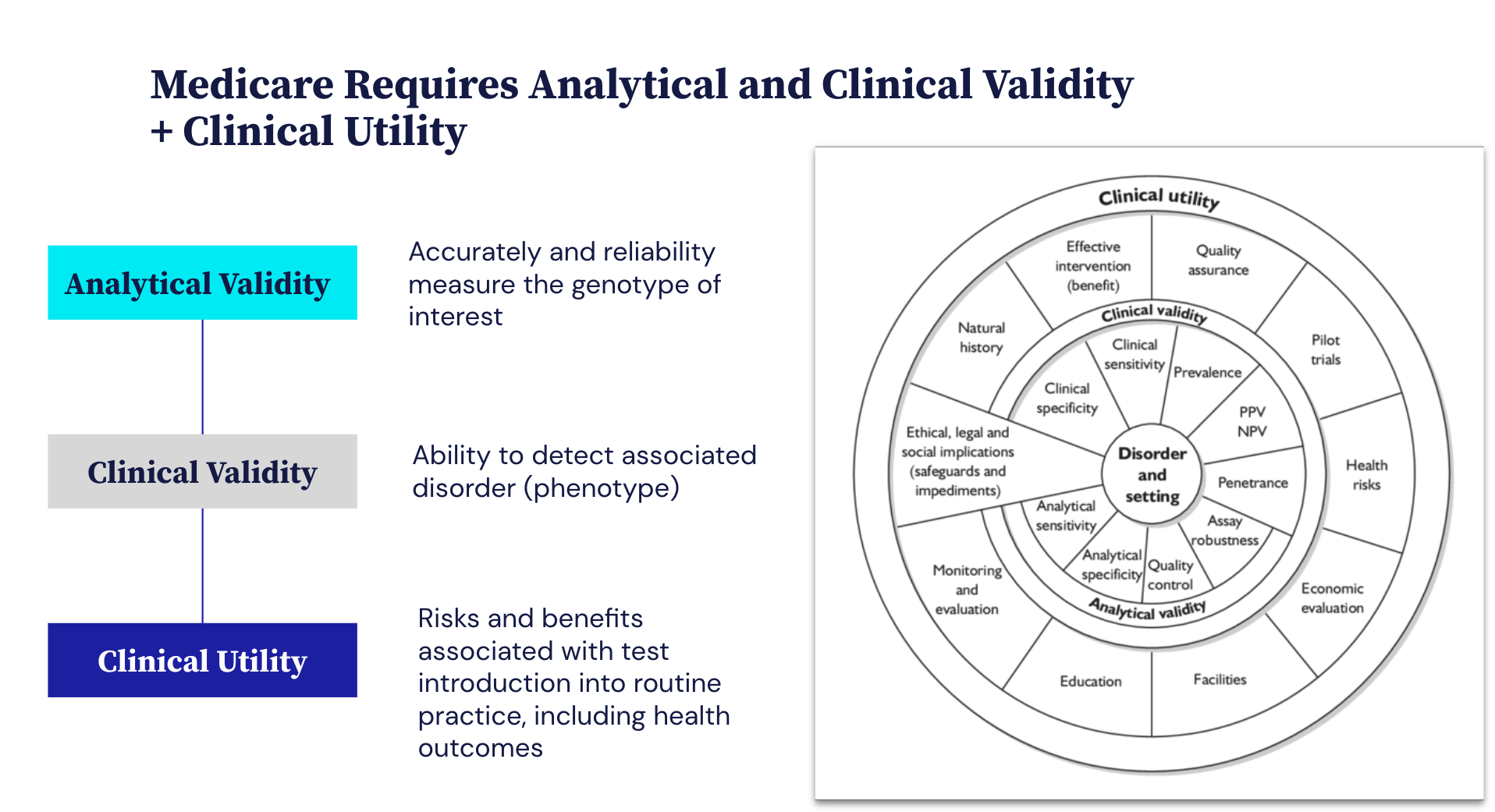
As NGS-based testing grows, NGS coverage and payment become critical drivers of adoption.




