Are you considering offering clinical NGS testing or looking for an economical approach to building your program?
If so, then you know that any eagerness to implement clinical NGS can quickly turn to discussions of all of the challenges involved. These challenges include everything from a scarcity of informatics expertise, to validating clinical testing protocols, to the time and expense of implementation.
Recently, we hosted a webinar on the CAP Distributive Model. What is the CAP Distributive Model? To describe the model, it’s helpful to consider the entire NGS testing workflow.
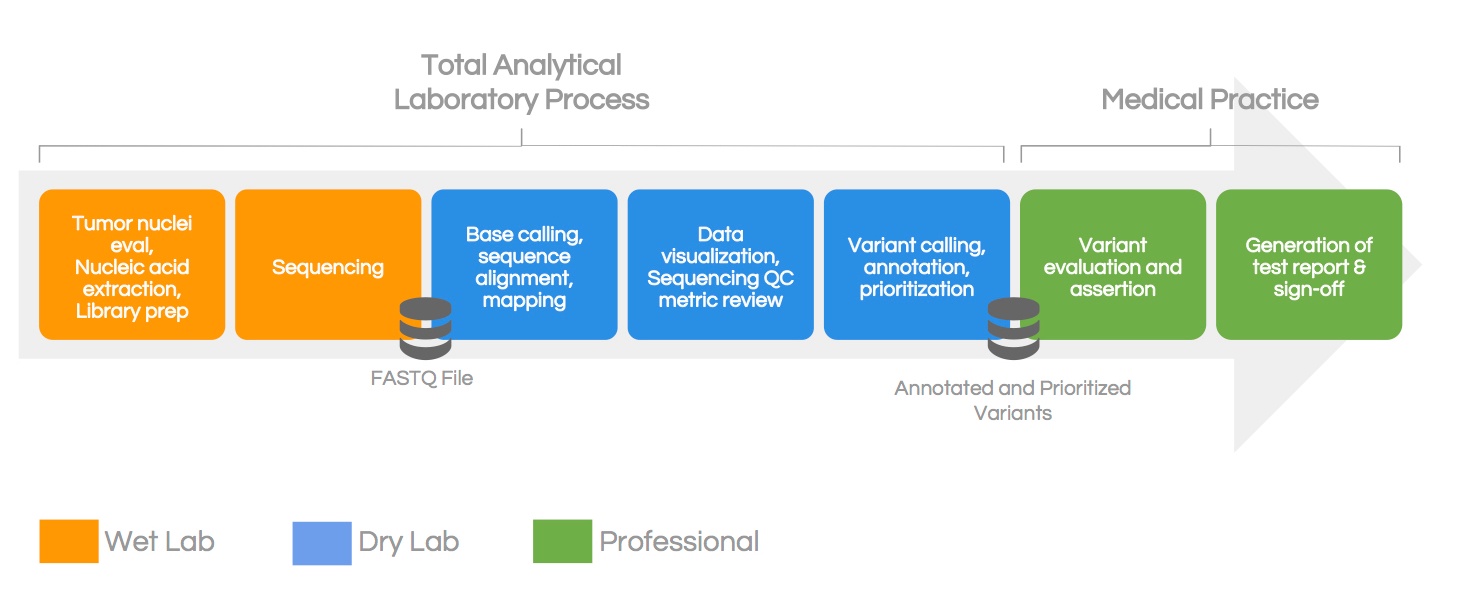
In this workflow, you can break down all of the process steps into one of three phases:
-Wet lab phase — sample accessioning, nucleic acid evaluation, sample and library prep, and sequencing
-Dry lab phase — variant calling, variant annotation and classification, data visualization, and quality control analysis
-Professional phase — clinical interpretation and reporting and final report and sign out from a certified medical director
The CAP Distributive Model states that any CLIA-certified or CAP-accredited lab is allowed to outsource any of the three components (wet lab, dry lab, or professional) to another CLIA-certified or CAP-accredited lab. Using this model can enable your lab to quickly and economically build or scale your NGS testing program.
To learn more about the CAP Distributive Model, its benefits, and the requirements for implementing it, download this recording of our recent webinar.


